Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Feeding Intolerance in Newborns
*Corresponding author: Lilach Hofi, Department of Neonatology, Kaplan Medical Center, Rehovot, Israel.
Received: April 25, 2022; Published: May 05, 2022
DOI: 10.34297/AJBSR.2022.16.002207
Abstract
Introduction: Feeding intolerance defined as the inability to digest enteral feedings, and is associated with increased gastric
residuals, abdominal distension and/or regurgitations. Recently, we observed in our neonatal intensive care unit, a tendency for
feeding intolerance in relation to origin.
Aim: To evaluate whether feeding intolerance is more prevalent in African Israelis newborns (AIN) versus other Israelis newborns
(OIN) origin.
Methods: A retrospective case control study based on medical records between 2014 and 2016. The groups included 50 AIN and
100 OIN.
Results: The AIN had significantly higher prevalence of feeding intolerance in (34%) compared to OIN (15%) (p=0.007). Moreover,
feeding intolerance symptoms were higher in the AIN group compared to the OIN group; gastric residual volume more than 50% (24%
vs 10%, p=0.014) regurgitations (76% vs 50%, p=0.002), vomiting (44% vs 10%, p=0.046), and two days without defecation (10% vs
2%, p=0.029). Mother’s own milk feeding was more prevalent in the AIN group.
There were no differences between the two groups in the prevalence of necrotizing enterocolitis, intestinal perforation, or milk
allergy.
Conclusion: Feeding intolerance was more prevalent in AIN versus OIN. Our research results can be used as a basis for future
studies on this population of newborns with feeding intolerances, examining the use of hypoallergenic formulas as a rescue treatment
or as a first-line treatment for the initial infancy period.
Keywords: Feeding intolerance, Hydrolyzed protein formula, Milk allergy, Newborns, Origin
Abbreviations: CMA: Cow’s Milk Allergy; DHM: Donor Human Milk; FI: Feeding Intolerance; GRV: Gastric Residual Volume; HPF: Hydrolyzed Protein Formula; MOM: Mother Own Milk; NEC: Necrotizing Enterocolitis; NICU: Neonatal Intensive Care Unit; SIP: Spontaneous Intestinal Perforation; VLBW: Very Low Birth Weight (<1500gr)
Background
Feeding intolerance (FI), defined as the inability to digest enteral feedings, presents as gastric residual volume (GRV) of more than 50%, abdominal distension and/or regurgitations [1]. The most common cause of feeding intolerance is low gut motility due to prematurity. Other pathophysiological mechanisms are possibly related to enzymatic digestion, hormonal responses, bacterial colonization, and local immunity [2]. FI often seen in newborns admitted to the neonatal intensive care unit (NICU) and can lead to enteral feed cessation, impaired nutrients supply, unnecessary blood tests and abdominal x-rays, to potential hospital-acquired infections due to a central inserted catheter and to long-term parenteral nutrition [2].
Mother’s own milk (MOM) is the best feeding choice for both term and preterm infants. MOM’s unique composition of digestive enzymes, growth factors, hormones, and prebiotics, offers clear benefits for the immature gastrointestinal functions and protective effects against gut microbiota alterations [2-4]. Moreover, there is evidence that a human milk-based diet is associated with better feeding tolerance in very-low-birthweight (VLBW) infants [5].
Preterm formulas that contain intact proteins are indicated for enteral feeding of preterm infants when sufficient MOM and donor human milk (DHM) are not available [6]. Hydrolyzed protein formulas (HPFs) have been shown to accelerate gastric emptying, increase stool frequency and reduce the time to full enteral feeds in preterm infants [7]. In addition, HPFs have been tested as an alternative strategy in treating FI in VLBW infants compared to standard preterm formulas and were shown to be beneficial [8]. However, formulas used in these studies were lactose free making it impossible to separate the effects of the hydrolyzed protein from other changes in the formula composition [9,10].
Former studies have found differences in lactose intolerance in newborns of African origin versus other origin newborns [11- 19]. Recently, we observed in our NICU, a tendency for FI in African Israelis newborns (AIN) compared to other Israelis newborns (OIN). Therefore, we aimed to evaluate whether feeding intolerance is more prevalent in AIN versus OIN origin that were formula-fed (>20% of daily volume) during their first week of life.
Methods
This is a retrospective case control study, based on the analysis of data collected from medical records of term and preterm infants admitted to Kaplan Medical Center’s (KMC) NICU between January 2014 and December 2016. The study group included 50 AIN and 100 OIN as controls. A match was made between the preterm infants in the two groups based on birth week (± 1-2 weeks) and birth weight (± 100-200 grams). Exclusion criteria included: death or discharge before the age of three days, congenital malformation, or asphyxia. We also excluded newborns that were exclusively MOM-fed (>80% of daily volume) [20]. Collected data included demographic data, anthropometric parameters, morbidities, medical treatments and feeding intolerance parameters (GRV, regurgitations, vomiting, frequency of stool), type of feeding (MOM versus formula and type of formula).
The KMC NICU follows a feeding protocol (appendix) based on the European Society for Paediatric Gastroenterology, Hepatology and Nutrition’s (ESPHAGAN) recommendations of parenteral and enteral nutrition [21]. The feeding protocol was not changed during the study observation period. There was no change in senior physician or dietitian. The sample size was calculated according to 50% frequency of FI in AIN and 16% frequency in OIN with 80% power and a significance level of p<0.05. The sample size was calculated to be 34 AIN and 68 OIN. Based on this data we included 50 AIN and 100 OIN. The study was approved by the Ethics Committee of KMC (0175-17-KMC).
Definitions
FI was defined by gastric residuals (GR), with or without regurgitations, vomiting, abdominal distention, low frequency of defecation and the disruption of the patient’s feeding plan [1]. Necrotizing enterocolitis (NEC) was defined according to the BELL criteria [22]. Cow’s milk allergy (CMA) was clinically defined by a. bloody stools without pathological laboratory or imagery findings except for eosinophilia or anemia in complete blood counts, and b. both negative stool cultures and viral pathogens in polymerase chain reactions and c. clinical improvement with the elimination of cow’s milk protein from the diet [23]. Spontaneous intestinal perforation (SIP) was defined as a neonate presenting typical clinical features of the disease (abdominal distension) with radiologically confirmed pneumoperitoneum, without radiographic evidence of NEC and when surgical data was available, SIP was confirmed when there was presence of isolated focal intestinal perforations surrounded by a normal-appearing bowel [24].
Statistical Analysis
Data is presented as median (range) or percentages. Continuous variables between the various study groups were tested for normality by a Shapiro-Wilk test and when abnormal distributions were found, non-parametric tests were performed. The Mann- Whitney test was performed to compare the two groups. Pearson chi-square tests were used for the relationship between two categorical variables. P values <0.05 were considered statistically significant. Data were analyzed using the Statistical Package for the Social Sciences (SPSS, software version 25, Chicago, IL, USA).
Results
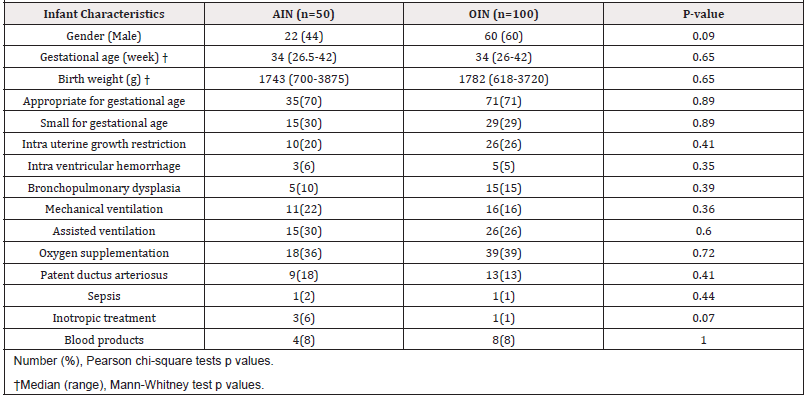
The study population consisted of 50 AIN and 100 OIN. There were no significant statistical differences between two groups in infant characteristics including anthropometric parameters or neonatal comorbidities (Table 1).
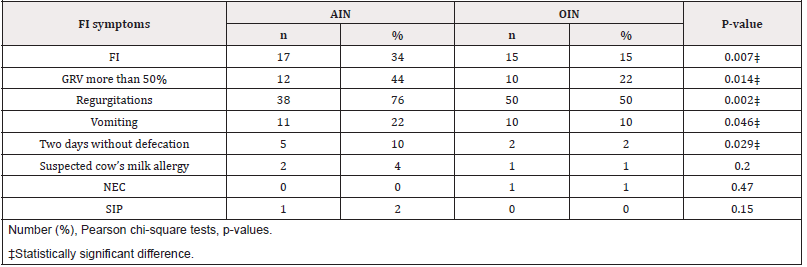
We found that the AIN group had significantly higher prevalence of FI compared to the OIN group (17/50, 34% vs 15/100, 15%, p=0.007). Moreover, the FI symptoms were higher in the AIN group compared to the OIN group; GRV more than 50% (12/50, 24% vs 10/100, 10%, p=0.014) regurgitations (38/50, 76% vs 50/100, 50%, p=0.002), vomiting (11/50, 44% vs 10/100, 10%, p=0.046), and two days without defecation (5/50, 10% vs 2/100, 2%, p=0.029).There were no differences in the prevalence of NEC, intestinal perforation, or CMA between the two groups (Table 2). MOM feeding (any volume) was more prevalent in the AIN group in the first week of life (12% vs 3% p=0.03), with 88% of the AIN and 97% of the OIN being formula-fed exclusively (>80% of daily volume) in the first week of life.
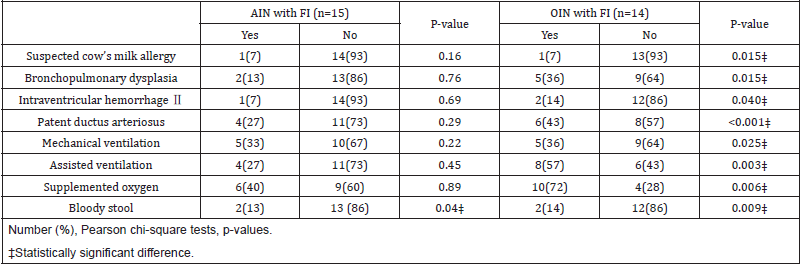
OIN with FI had higher rates of other neonatal comorbidities and respiratory support compared to OIN without FI. This association was not found in the AIN group (Table 3). The prevalence of changing the cow’s milk intact protein-based formula to HPFs was higher in the AIN group compared to the OIN group (14/50, 28% vs 8/100, 8%, respectively, p=0.001). The prevalence of clinical improvement in FI after formula change was higher in the AIN group, though not statistically significant (12/14 86% vs 5/8 62%, p=0.21). The weight at discharge was significantly lower in the AIN group than in the OIN group (2349±476g vs 2479±473g, respectively, p=0.036).
Discussion
Our retrospective case control study demonstrated that FI is more prevalent in AIN compared to OIN. There is evidence that human-milk-based diet is associated with better feeding tolerance in VLBW infants [5]. We aimed to study FI in AIN versus OIN that were formula-fed during their first week of life, as a basis for a future study investigating HPFs as a rescue or as a first line feeding in AIN not receiving MOM. Although MOM feeding in the first week of life was more prevalent in the AIN group, they still had a higher rate of FI. These results might indicate that the origin of the newborns may play a role in the presence of feeding intolerance.
Furthermore, there were no differences in comorbidities in the two groups. The discharged weight of the AIN group was significantly lower than the OIN group. We assume that lower weight is secondary to the higher rate of FI as abnormal weight gain is a known complication of FI [2].
OIN with FI had higher rates of other neonatal comorbidities and respiratory support compared to OIN without FI. This suggests that FI might be secondary to other morbidities in the OIN group. This association was not found in the AIN group.
We speculate that FI is associated with an intrinsic factor in the AIN group, such as lactose intolerance or differences in genetics and/or the microbiome. The higher prevalence of clinical improvement in AIN given HPFs compared to OIN also supports this hypothesis, as HPFs are also lactose free unlike MOM that contains lactose [25]. There were several studies in the 1960s and 1970s suggesting a higher prevalence of lactose intolerance in African descent infants [10-18]. These studies included children over one year old. They theorized that there is a genetic tendency of lactose intolerance in this ethnic decent. Another theory suggested that this is an acquired tendency, secondary to low consumption of milk products. Another theoretical explanation is differences in the microbiome between the two ethnic groups. Recently, Yuan et al. found that the gut microbiota composition of preterm infants diagnosed with feeding intolerance was significantly different than that of preterm infants without feeding intolerance. They suggest that the abundance of Klebsiella may cause feeding intolerance [26].
Limitations
The main limitation of the study relates to its retrospective design and a relatively small study group number. Additionally, FI is a clinical diagnosis with an equivocal definition, which can be confused with other clinical diagnosis.
Conclusion
FI was more prevalent in AIN versus the OIN group. The origin of the newborn may be another factor that influences the development of FI. Early identification of risk factors for FI may help to suit better nutritional therapy. To the best of our knowledge, this is the first study examining this issue. Further studies are needed to determine whether the difference is due to lactase deficiency or microbiome variability.
Individualized Contributions
Dr Calanit Hershkovich–Shporen was responsible for the conceptualization, methodology, investigation, validation, original draft preparation. Ms Lilach Hofi was responsible for conceptualization, methodology and writing-review and editing. Dr Alona Khodajev was responsible for data curation.Pro. Orna Flidel- Rimon was responsible methodology, writing-review, and editing.
Acknowledgements
None.
Conflict of Interest
None.
References
- Moore TA, Wilson ME (2011) Feeding intolerance: a concept analysis. Adv Neonatal Care 11(3): 149-154.
- Fanaro S (2013) Feeding intolerance in the preterm infant. Early Human Development 89: S13-S20.
- American Academy of Pediatrics Section on Breastfeeding (2012) Breastfeeding and the use of human milk. Pediatrics 129(3): 827-841.
- Ford SL, Lohmann P, Preidis GA, Gordon PA, O’Donnell A, et al. (2019) Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother’s own milk compared with donor breast milk. Am J Clin Nutr 109: 1088-1097.
- Sisk PM, Lovelady CA, Gruber KJ, Dillard RG, O’Shea TM (2008) Human milk consumption and full enteral feeding among infants who weight < 1250 grams. Pediatrics 121(6): 1528-1533.
- Hay Jr W, Hendrickson KC (2017) Preterm formula use in the preterm very low birth weight infant. Seminars in Fetal & Neonatal Medicine 22: 15-22.
- Mihatsch WA, Franz AR, Hogel J, Pohlandt F (2002) Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 110: 1199-1203.
- Raimondi F, Sepra AM, Sellitto M, Landolfo F, Capasso L, et al. (2012) Amino acid-based formula as a rescue strategy in feeding very low birth weight infants with intrauterine growth restriction. J Pediatr Gastroenterol Nutr 54(5): 608-612.
- Senterre T, Rigo J (2016) Hydrolyzed Proteins in Preterm Infants, Protein in Neonatal and Infant Nutrition: Recent Updates. Nestlé Nutr Inst Workshop 86: 39-49.
- Fleischer DM, Venter C, Vandenplas Y (2016) Hydrolyzed Formula for Every Infant? Protein in Neonatal and Infant Nutrition: Recent Updates. Nestlé Nutr Inst Workshop 86: 51-65.
- Paige DM, Bayless TM, Mellits DE, Davis L (1977) Lactose malabsorption in preschool black children. Am J Clin Nutr 30: 1018-1022.
- Bayless TM, Rosensweig NS (1966) A Racial Difference in Incidence of Lactase Deficiency: a survey of milk intolerance and lactase deficiency in healthy adult males JAMA 197(12): 968-972.
- Cook GC, Kajubi SK (1966) Tribal Incidence of Lactase Deficiency in Uganda. Lancet 2: 725-729.
- Segal I, Gagjee PP, Essop AR, Noormohamed AM (1983) Lactase deficiency in the South African black population. Am J Clin Nutr 38 (6): 901-905.
- Olatunbosun DA, Kwaku AB (1972) Lactose intolerance in Nigerian children. Acta Pediat Scand 61: 715-719.
- Bowie MD, Brinkman GL, Hansen JDL (1965) Acquired disaccharide intolerance in malnutrition. J Paediatr 66: 1083-1091.
- Jersky J, Kinsley RH (1967) Lactase deficiency in the South African Buntu. S Afr Med J 41: 1194-1196.
- Welsh JD, Zschiesche OM, Willits VL, Rassell L (1968) Studies of lactose intolerance in families. Arch Intern Med 122 (4): 315-317.
- (1972) Lactase activity levels in Nigeria- Genetic or Acquired phenomenon? Nutrition Reviews 30(7): 156-158.
- Cormack BE, Embleton ND, Van Goudoever JB, Hay WW, Bloomfield FH, et al. (2016) Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatr Res Jun79(6): 810-820.
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, et al. (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr Jan50(1): 85-91.
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, et al. (1978) Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg 187: 1-7.
- Akta S, Ergenekon E, Unal S, Turkylmaz C, Hirfanoglu IM, et al. (2017) Different presentations of cow's milk protein allergy during neonatal period. Turk J Pediatr 59: 322-328.
- Gebus M, Michel J, Samperiz S, Harper L, Alessandri J, et al. (2018) Mangement of neonatal spontaneous intestinal perforation by peritoneal needle aspiration. J Perinatol 38 (2): 159-163.
- Mimouni FB, Lubetzky R, Yochpaz S, Mandel D (2017) Preterm human milk macronutrient and energy composition. A systemic review and meta-analysis. Clin Perinatol 44: 165-172.
- Yuan Z, Yan J, Wen H, Deng X, Li X, et al. (2019) Feeding intolerance alters the gut microbiota of preterm infants. PLoS One 14(1): e0210609.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.